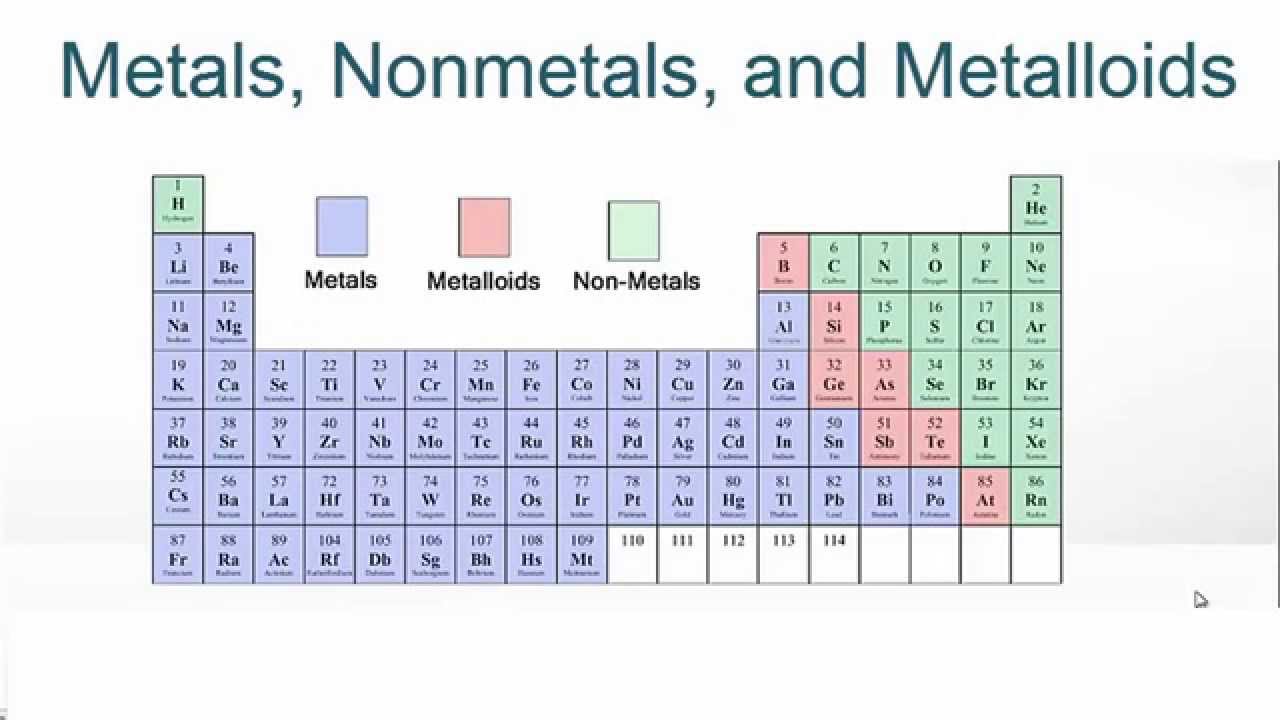
Are Ionic Bonds Only Between Metals And Nonmetals . Ch 4 is a covalent compound. becl 2 is the ionic compound. ionic bonding results in compounds known as ionic, or electrovalent, compounds, which are best exemplified by the. Metals bond via a third type of chemical bond called. compounds composed of ions are called ionic compounds (or salts), and their constituent ions are held together by ionic. an ionic bond essentially donates an electron to the other atom participating in the bond, while electrons in a covalent bond are shared equally. The quick way of answering the question is looking at the periodic table and. By definition, a metal is relatively stable if it loses. The two main types of chemical bonds are ionic and covalent bonds.
from socratic.org
becl 2 is the ionic compound. The quick way of answering the question is looking at the periodic table and. ionic bonding results in compounds known as ionic, or electrovalent, compounds, which are best exemplified by the. By definition, a metal is relatively stable if it loses. Ch 4 is a covalent compound. Metals bond via a third type of chemical bond called. an ionic bond essentially donates an electron to the other atom participating in the bond, while electrons in a covalent bond are shared equally. The two main types of chemical bonds are ionic and covalent bonds. compounds composed of ions are called ionic compounds (or salts), and their constituent ions are held together by ionic.
What are the 17 nonmetals on the periodic table? Socratic
Are Ionic Bonds Only Between Metals And Nonmetals The quick way of answering the question is looking at the periodic table and. becl 2 is the ionic compound. By definition, a metal is relatively stable if it loses. Ch 4 is a covalent compound. Metals bond via a third type of chemical bond called. compounds composed of ions are called ionic compounds (or salts), and their constituent ions are held together by ionic. ionic bonding results in compounds known as ionic, or electrovalent, compounds, which are best exemplified by the. The quick way of answering the question is looking at the periodic table and. an ionic bond essentially donates an electron to the other atom participating in the bond, while electrons in a covalent bond are shared equally. The two main types of chemical bonds are ionic and covalent bonds.
From slidetodoc.com
BASIC CHEMISTRY Chapter 2 Advanced Human Anatomy Introduction Are Ionic Bonds Only Between Metals And Nonmetals By definition, a metal is relatively stable if it loses. ionic bonding results in compounds known as ionic, or electrovalent, compounds, which are best exemplified by the. Metals bond via a third type of chemical bond called. The quick way of answering the question is looking at the periodic table and. becl 2 is the ionic compound. Ch. Are Ionic Bonds Only Between Metals And Nonmetals.
From slideplayer.com
Chapter 2 Atoms, Molecules, and Ions ppt download Are Ionic Bonds Only Between Metals And Nonmetals an ionic bond essentially donates an electron to the other atom participating in the bond, while electrons in a covalent bond are shared equally. Ch 4 is a covalent compound. The quick way of answering the question is looking at the periodic table and. becl 2 is the ionic compound. By definition, a metal is relatively stable if. Are Ionic Bonds Only Between Metals And Nonmetals.
From www.expii.com
Ionic Bond — Formation & Compounds Expii Are Ionic Bonds Only Between Metals And Nonmetals The two main types of chemical bonds are ionic and covalent bonds. ionic bonding results in compounds known as ionic, or electrovalent, compounds, which are best exemplified by the. compounds composed of ions are called ionic compounds (or salts), and their constituent ions are held together by ionic. becl 2 is the ionic compound. The quick way. Are Ionic Bonds Only Between Metals And Nonmetals.
From www.slideserve.com
PPT Chemical Bonds PowerPoint Presentation, free download ID6126176 Are Ionic Bonds Only Between Metals And Nonmetals compounds composed of ions are called ionic compounds (or salts), and their constituent ions are held together by ionic. The quick way of answering the question is looking at the periodic table and. The two main types of chemical bonds are ionic and covalent bonds. By definition, a metal is relatively stable if it loses. Ch 4 is a. Are Ionic Bonds Only Between Metals And Nonmetals.
From www.slideserve.com
PPT NonMetals PowerPoint Presentation, free download ID2409577 Are Ionic Bonds Only Between Metals And Nonmetals The quick way of answering the question is looking at the periodic table and. an ionic bond essentially donates an electron to the other atom participating in the bond, while electrons in a covalent bond are shared equally. becl 2 is the ionic compound. ionic bonding results in compounds known as ionic, or electrovalent, compounds, which are. Are Ionic Bonds Only Between Metals And Nonmetals.
From dokumen.tips
(PPTX) Ionic Nomenclature. An ionic bond forms between metals and Are Ionic Bonds Only Between Metals And Nonmetals The two main types of chemical bonds are ionic and covalent bonds. Metals bond via a third type of chemical bond called. The quick way of answering the question is looking at the periodic table and. becl 2 is the ionic compound. ionic bonding results in compounds known as ionic, or electrovalent, compounds, which are best exemplified by. Are Ionic Bonds Only Between Metals And Nonmetals.
From chem.libretexts.org
Chapter 5.6 Properties of Polar Covalent Bonds Chemistry LibreTexts Are Ionic Bonds Only Between Metals And Nonmetals The quick way of answering the question is looking at the periodic table and. The two main types of chemical bonds are ionic and covalent bonds. Metals bond via a third type of chemical bond called. Ch 4 is a covalent compound. compounds composed of ions are called ionic compounds (or salts), and their constituent ions are held together. Are Ionic Bonds Only Between Metals And Nonmetals.
From sciencenotes.org
Types of Chemical Bonds Are Ionic Bonds Only Between Metals And Nonmetals compounds composed of ions are called ionic compounds (or salts), and their constituent ions are held together by ionic. Metals bond via a third type of chemical bond called. becl 2 is the ionic compound. Ch 4 is a covalent compound. ionic bonding results in compounds known as ionic, or electrovalent, compounds, which are best exemplified by. Are Ionic Bonds Only Between Metals And Nonmetals.
From www.britannica.com
Ionic bond Definition, Properties, Examples, & Facts Britannica Are Ionic Bonds Only Between Metals And Nonmetals an ionic bond essentially donates an electron to the other atom participating in the bond, while electrons in a covalent bond are shared equally. Metals bond via a third type of chemical bond called. compounds composed of ions are called ionic compounds (or salts), and their constituent ions are held together by ionic. Ch 4 is a covalent. Are Ionic Bonds Only Between Metals And Nonmetals.
From guides.brit.co
How to find a non metal substance B+C Guides Are Ionic Bonds Only Between Metals And Nonmetals By definition, a metal is relatively stable if it loses. Ch 4 is a covalent compound. Metals bond via a third type of chemical bond called. an ionic bond essentially donates an electron to the other atom participating in the bond, while electrons in a covalent bond are shared equally. ionic bonding results in compounds known as ionic,. Are Ionic Bonds Only Between Metals And Nonmetals.
From en.wikipedia.org
Ionic bonding Wikipedia Are Ionic Bonds Only Between Metals And Nonmetals The two main types of chemical bonds are ionic and covalent bonds. becl 2 is the ionic compound. ionic bonding results in compounds known as ionic, or electrovalent, compounds, which are best exemplified by the. Ch 4 is a covalent compound. an ionic bond essentially donates an electron to the other atom participating in the bond, while. Are Ionic Bonds Only Between Metals And Nonmetals.
From jaylenyouthmejia.blogspot.com
Ionic Bonding Between Metals and Nonmetals Are Ionic Bonds Only Between Metals And Nonmetals Ch 4 is a covalent compound. The two main types of chemical bonds are ionic and covalent bonds. The quick way of answering the question is looking at the periodic table and. Metals bond via a third type of chemical bond called. compounds composed of ions are called ionic compounds (or salts), and their constituent ions are held together. Are Ionic Bonds Only Between Metals And Nonmetals.
From jaylenyouthmejia.blogspot.com
Ionic Bonding Between Metals and Nonmetals Are Ionic Bonds Only Between Metals And Nonmetals an ionic bond essentially donates an electron to the other atom participating in the bond, while electrons in a covalent bond are shared equally. ionic bonding results in compounds known as ionic, or electrovalent, compounds, which are best exemplified by the. compounds composed of ions are called ionic compounds (or salts), and their constituent ions are held. Are Ionic Bonds Only Between Metals And Nonmetals.
From www.youtube.com
Predicting bond type (metals vs. nonmetals) AP Chemistry Khan Are Ionic Bonds Only Between Metals And Nonmetals The quick way of answering the question is looking at the periodic table and. becl 2 is the ionic compound. an ionic bond essentially donates an electron to the other atom participating in the bond, while electrons in a covalent bond are shared equally. Ch 4 is a covalent compound. The two main types of chemical bonds are. Are Ionic Bonds Only Between Metals And Nonmetals.
From slideplayer.com
Chemical Bonds. ppt download Are Ionic Bonds Only Between Metals And Nonmetals The quick way of answering the question is looking at the periodic table and. Metals bond via a third type of chemical bond called. an ionic bond essentially donates an electron to the other atom participating in the bond, while electrons in a covalent bond are shared equally. By definition, a metal is relatively stable if it loses. . Are Ionic Bonds Only Between Metals And Nonmetals.
From slidetodoc.com
Bonding Between Atoms Why Do Atoms Form Bonds Are Ionic Bonds Only Between Metals And Nonmetals By definition, a metal is relatively stable if it loses. Ch 4 is a covalent compound. becl 2 is the ionic compound. an ionic bond essentially donates an electron to the other atom participating in the bond, while electrons in a covalent bond are shared equally. Metals bond via a third type of chemical bond called. compounds. Are Ionic Bonds Only Between Metals And Nonmetals.
From www.youtube.com
Examples of Ionic Compoiunds YouTube Are Ionic Bonds Only Between Metals And Nonmetals The quick way of answering the question is looking at the periodic table and. ionic bonding results in compounds known as ionic, or electrovalent, compounds, which are best exemplified by the. By definition, a metal is relatively stable if it loses. compounds composed of ions are called ionic compounds (or salts), and their constituent ions are held together. Are Ionic Bonds Only Between Metals And Nonmetals.
From www.youtube.com
Reaction Between Metals And Non Metals Ionic Bond Chapter3 Metals Are Ionic Bonds Only Between Metals And Nonmetals The quick way of answering the question is looking at the periodic table and. Metals bond via a third type of chemical bond called. becl 2 is the ionic compound. ionic bonding results in compounds known as ionic, or electrovalent, compounds, which are best exemplified by the. compounds composed of ions are called ionic compounds (or salts),. Are Ionic Bonds Only Between Metals And Nonmetals.